Steadying the Ship: Regulators’ Fight Against Health-Care Fraud
By Samuel May Aug 11, 2022
By Samuel May Aug 11, 2022
In July 2022, the United States Department of Justice, Office of the Attorney General (OAG) and the US Department of Health and Human Services, Office of the Secretary, released its Health Care Fraud and Abuse Control Program Annual Report for Fiscal Year 2021.
The yearly report summarizes the actions of the Health Care Fraud and Abuse Control Program (HCFAC) established by the Health Insurance Portability and Accountability Act of 1996 (HIPAA). The program was designed to coordinate federal, state and local law enforcement activities on health care fraud and abuse.
Key takeaways from the report:
In comparison to previous years, the total dollar value of health care fraud findings saw a dramatic increase from $1.8 billion in FY 2020. The funds recovered and returned to the federal government and the Medicare Trust Fund fell behind the previous two years but will likely see an upswing in future reports based on the new outstanding judgments and settlements. Exclusions have been in steady decline from FY 2018, with FY 2021 seeing the fewest total exclusions. Criminal convictions also saw a marked decline in the last three reports. The charts below summarize some of the reported figures over the last four annual reports:
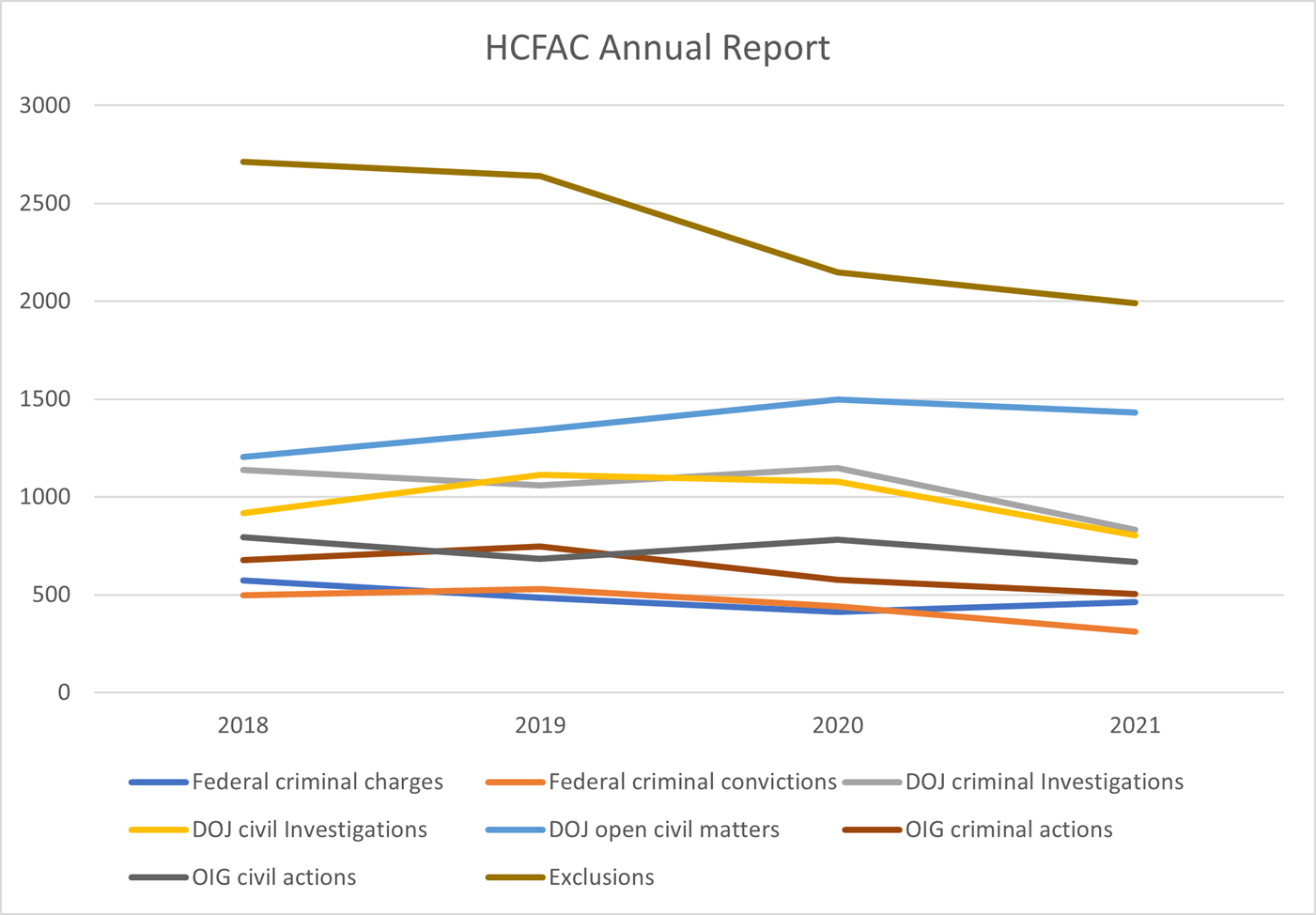
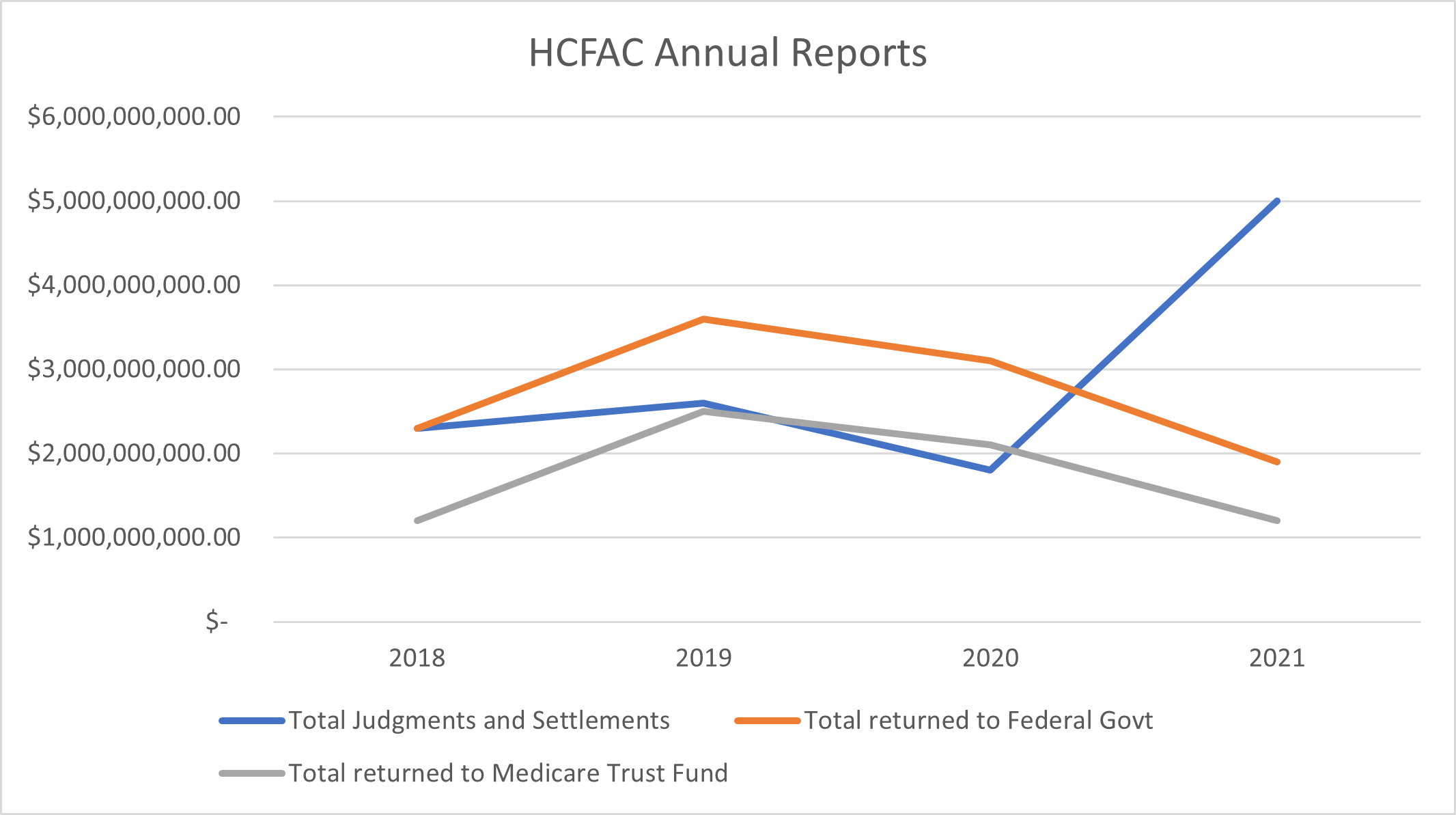
ANOTHER REPORT
The Department of Health and Human Services Office of Inspector General (HHS-OIG) also recently released its Semiannual Report to Congress. The most recent report as of this article publication was for October 1, 2021 through March 31, 2022. During this reporting period, the HHS-OIG issued 47 audit reports and 14 evaluation reports, identifying $1.14 billion in expected audit recoveries and $1.6 billion in “questioned” costs. The HHS-OIG also made 130 new recommendations to HHS that it said could save a potential $162.1 million. On the investigative side, the HHS-OIG (in conjunction with the DOJ, Medicaid Fraud Control Units and other law enforcement agencies) expects approximately $1.44 billion in investigative recoveries and 320 criminal actions. In total, the report “highlights nearly $3 billion in expected recoveries as a result of HHS-OIG audits and investigations.” In terms of efficiency, the HHS-OIG claimed that for every $1 spent on the Health Care Fraud and Abuse Control Program, more than $4 was recovered.
On specific health care fronts, the HHS-OIG included in the report its intended response to the presidential initiative aimed at improving nursing home quality and safety. Officials state they will expand their nursing home oversight and deploy a new strategy to better combat what they call “widespread and persistent problems.” The new initiative was announced on March 1, 2022, by President Joseph R. Biden at the State of the Union address and was prefaced by a fact sheet issued by the White House on February 28, 2022. The agenda, entitled “Protecting Seniors and People with Disabilities by Improving Safety and Quality of Care in the Nation’s Nursing Homes,” focuses on nursing home staff, nursing home accountability and more transparency into nursing home conditions.
MOVING FORWARD
On July 20, 2022, the United States Department of Justice (DOJ) released information about dozens of fraud charges totaling $1.2 billion in health care fraud schemes. The charges were spread across 13 federal districtsand 36 defendants, and included telemedicine, cardiovascular and genetic testing and durable medical equipment (DME) schemes. A large portion of this nationwide action was focused on a network of telemarketers who allegedly lured Medicare and Medicaid patients into participating in unnecessary testing. The telemarketers allegedly participated in the payment of illegal kickbacks and bribes with various laboratory owners in exchange for patient referrals. The telemedicine schemes accounted for $1 billion of the total alleged losses.
Telemedicine and laboratory testing schemes have grown in popularity as telehealth services have become more popular and widely utilized, especially in the height of the COVID-19 pandemic. An October 2021 Data Snapshot from the HHS-OIG found that 26 million Medicare beneficiaries (approximately 39% of the Medicare population) received telehealth services from March through December 2020. The HHS-OIG semiannual report found that, during the same period, 84% of those received telehealth services from providers they had an established relationship with. Such a large shift in usage will almost always create opportunities for bad actors to explore new fraud schemes as regulations and proper oversight lag behind.
For these telemedicine/laboratory schemes, the telemarketing company will target vulnerable groups of patients and convince them to see a telemedicine provider. The telemedicine provider will then create an order for the testing and send it to a laboratory owner or operator participating in the scheme. The laboratory conducts the test (sometimes properly but sometimes not) and bills the patient’s insurance. The laboratory makes its money on the claim submission, the telemarketing company will generally receive a kickback from the laboratory and the telemedicine providers receive bribes from the telemarketing company for creating the orders. The same form of scheme can also involve durable medical equipment (DME) companies where telemedicine providers create medically unnecessary DME orders instead of testing orders.
One scheme targeting Medicare and Veteran Affairs patients submitted $134 million in unnecessary testing and DME claimsand received $29 million in payments. Another similar scheme led to a charge of $73 million conspiracy for medically unnecessary cancer and cardiovascular genetic testing.
To further drive home the new focus on telehealth fraud, the HHS-OIG released a Special Fraud Alert on July 20, 2022 warning practitioners to exercise caution when dealing with telemedicine companies. In the alert, the HHS-OIG acknowledges the growing popularity of telehealth services and documents the various telehealth schemes described above. Both physicians and non-physicians are warned of the aggressive recruitment strategies the telemedicine companies have been utilizing, providing significant rewards (bribes) to practitioners for creating unnecessary orders. The HHS-OIG provides an “illustrative, not exhaustive” list of suspect characteristics:
U.S. federal regulators’ fight against health care fraud continues to expand and improve. Though it appears the pandemic might have negatively affected their efforts and opened new avenues for fraud schemes to spread, the recent reports and criminal and civil charges suggest the various agencies are aware of the problem and working to steady the ship. Though they combat a variety of different health care arenas, the focus for the immediate future appears to remain on telemedicine, unnecessary testing and nursing facility quality of care.